The dielectric constant refers to the degree to which a material can store electrical energy in an electrical field.
Additionally, materials that store electrical energy without allowing electric current to flow through them in an electric field are called dielectrics.
The dielectric constant is used to find materials with optimal dielectric and insulating properties under various conditions. It is particularly used in evaluating the performance of electronic components such as capacitors and insulating coatings.
In this article, we will provide a detailed explanation of the dielectric constant, including its measurement methods and thermal properties.
What is Dielectric Constant?
Insulators are materials that do not conduct direct current, such as polyvinyl chloride used in plastic or wire insulation. Conductors, on the other hand, are materials that conduct electricity like metals.
Now, there are materials that do not conduct direct current but they polarize in an alternating electric field and store electrical energy, these are called dielectrics. This can be illustrated with the example of a capacitor in an electronic circuit, as shown in the following diagram.
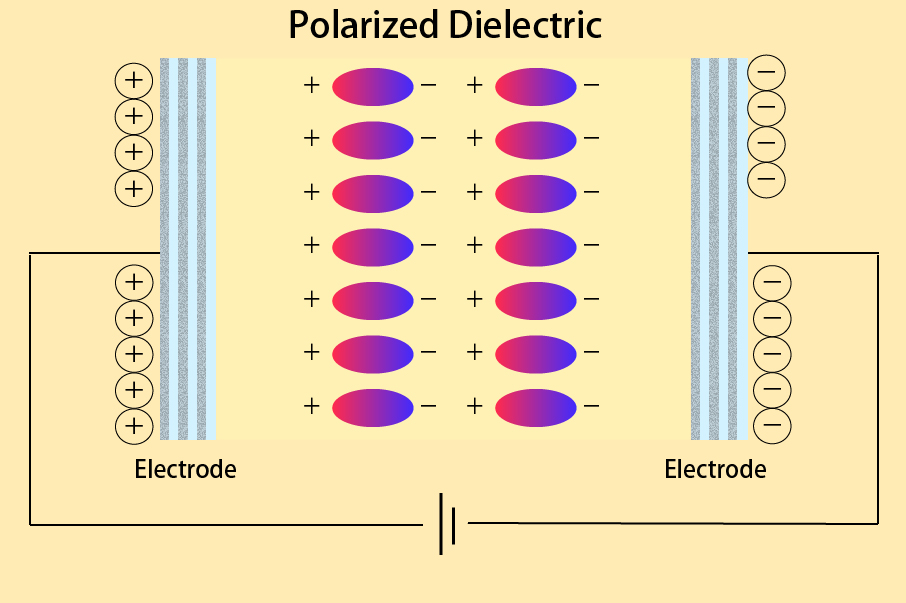
Based on the ceramic academy ‘Dielectric Materials Course 01‘
Electric charge is stored in the capacitor due to polarization, as shown in the figure. The degree of polarization varies depending on the material. This is where the dielectric constant becomes important.
Dielectrics and the Dielectric Constants of Key Materials

Even for insulators, materials that do not conduct direct current, they can become dielectrics when alternating current flows through them.
For example, aluminum oxide and tantalum oxide are dielectrics; they’re used in aluminum electrolytic capacitors and tantalum capacitors respectively. Barium titanate, polypropylene, mica, and other materials like these are also used as dielectrics in capacitors.
| Material | Capacitor Type | Features | Relative Permittivity* |
| Aluminum oxide | Aluminum electrolytic | Inexpensive, high capacitance | 8~10 |
| Tantalum oxide | Tantalum | Currently switching to ceramic capacitors | 23~27 |
| Barium titanate | Ceramic | Compact, high capacitance, wide variety | 5~500(Type 1.)200~100,000(Type 2.) |
| Polypropylene | Film | Balanced performance, commonly used in mechanical and electronic circuits | 2.1~2.2 |
| Mica | Mica | Heat resistant, no aging degradation | 6~8 |
| Paper (Cellulose) | Paper | Impregnated with silicone oil, mineral oil; alternates layers with metal foil. High insulation resistance | 3.5~65.5 |
*The ratio of the permittivity of a medium to the permittivity of a vacuum” (quoted from Wikipedia)
Dielectric Mechanisms
Dielectric polarization occurs primarily through four mechanisms: electronic polarization, ionic polarization, orientational polarization, and space charge polarization.
Here are the mechanisms for each type of polarization.
| Dielectric Mechanism | Occurrence Mechanism |
| Electronic Polarization | With an electric field applied, atoms get polarized, which causes electrons to shift. This shift allows for a dipole moment to develop. |
| Ionic Polarization | With an electric field applied, ions are polarized, leading to its shift and the formation of a dipole moment. |
| Orientational Polarization | Molecules with dipoles (molecules with a bias towards positive and negative charges within the molecule) rotate and align in response to the electric field. |
| Space Charge Polarization | Distribution of the charges within the material is altered due to an external electric field. This occurs at the interface of an electrode. |
Methods for Measuring Dielectric Constant
The measurement of permittivity involves several methods. Representative methods include the “concentration constant method,” “reflection-transmission method (S-parameter method),” and “resonance method.” Summarized in a table, they are as follows:
| MethodMeasurement | Description |
| Concentration Constant Method | Also known as capacitance method, the material is sandwiched between capacitor plates to measure the capacitance with and without the sample present. The dielectric constant is the ratio of these two values. |
| Reflection-Transmission Method (S-parameter method) | Also known as the waveguide method, the material is placed inside a waveguide (transmission line). The changes in the reflected and transmitted waves are measured to determine the dielectric constant. |
| Resonance Method | Also known as resonant line method, the material is placed in a resonant line, a circuit designed to resonate at specific frequencies. The material will cause changes in the resonant frequency values, with the arithmetic difference indicating the dielectric constant. |
Based on the investigation and research on measurement techniques and standard supply of dielectric constant and other material constants.
Conclusion
In this article, we explained the concept behind the dielectric constant, which quantitatively represents the ease of polarization of a material. It expresses the material’s extent to which it can store electricity (or be polarized). Materials that exhibit the ability to polarize in an alternating electric field and store electrical energy, even if they don’t conduct direct current, are dielectrics.
Permittivity arises from the arrangement of electrons and ions within a material, the electrical orientation of molecules within it, and the polarization caused by electron movement within the material. This is then used to evaluate the performance of electronic components such as capacitors and insulation coatings.
The “CrowdChem Data Platform” released by CrowdChem provides knowledge and information in the field of chemistry, including catalog information on products influencing permittivity and patent information associated with those products.
Some services are available for free, so please take advantage of them. For a free trial of the “CrowdChem Data Platform,” click below.